Our Technology
How it Works
The Re-2Ox process takes metal-rich mining concentrates, or recycled battery materials and converts them into premium battery materials.
Our Flow Sheet
Electric Vehicle Metals
Copper, Cobalt, Nickel
Byproduct Recovery
Arsenic, Bismuth, Antimony
Rare Earths
Cesium, Scandium, Rubidium
View Interview
Read Report
Directly from the Founder
Questions and Answers
An edited transcript from an interview has been summarized below
We're a group of older professionals who take over what we call distressed assets, distressed shells, and we try to make something out of them for our investors, and hopefully a major player comes in, takes us out after we've completed a fair amount of work. One of the assets we picked up in Canada's Cobalt Camp, and it's evolving, was the high-grade silver Castle Mine, which also has a lot of cobalt, nickel, copper, and arsenic. What we've done is develop an extraction process we call Re-2Ox.
Without this process, the whole Cobalt Camp, which in its day used to have about 108 mines, cannot produce cobalt sulphate or any battery metal, but the process itself has that ability, and we've already produced in lab tests a cobalt sulphate on what we call Asian specifications.
Basically, this is a process that was developed over a six-year timeframe and was originally designed to be working with what's called a primary metal, in other words, a concentrate from mined material in the Cobalt Camp. Over that six-year timeframe, we did a lot of bench-scale test work. We designed two pilot plants, then did full scale testing, and we produced, at that time, a cobalt carbonate. The market wanted a cobalt carbonate, so we produced a cobalt carbonate, and we were on spec.
Moving forward, conditions change, demands change, so now the market wants cobalt sulphate. So, we took the process and we produced cobalt sulphate with it.
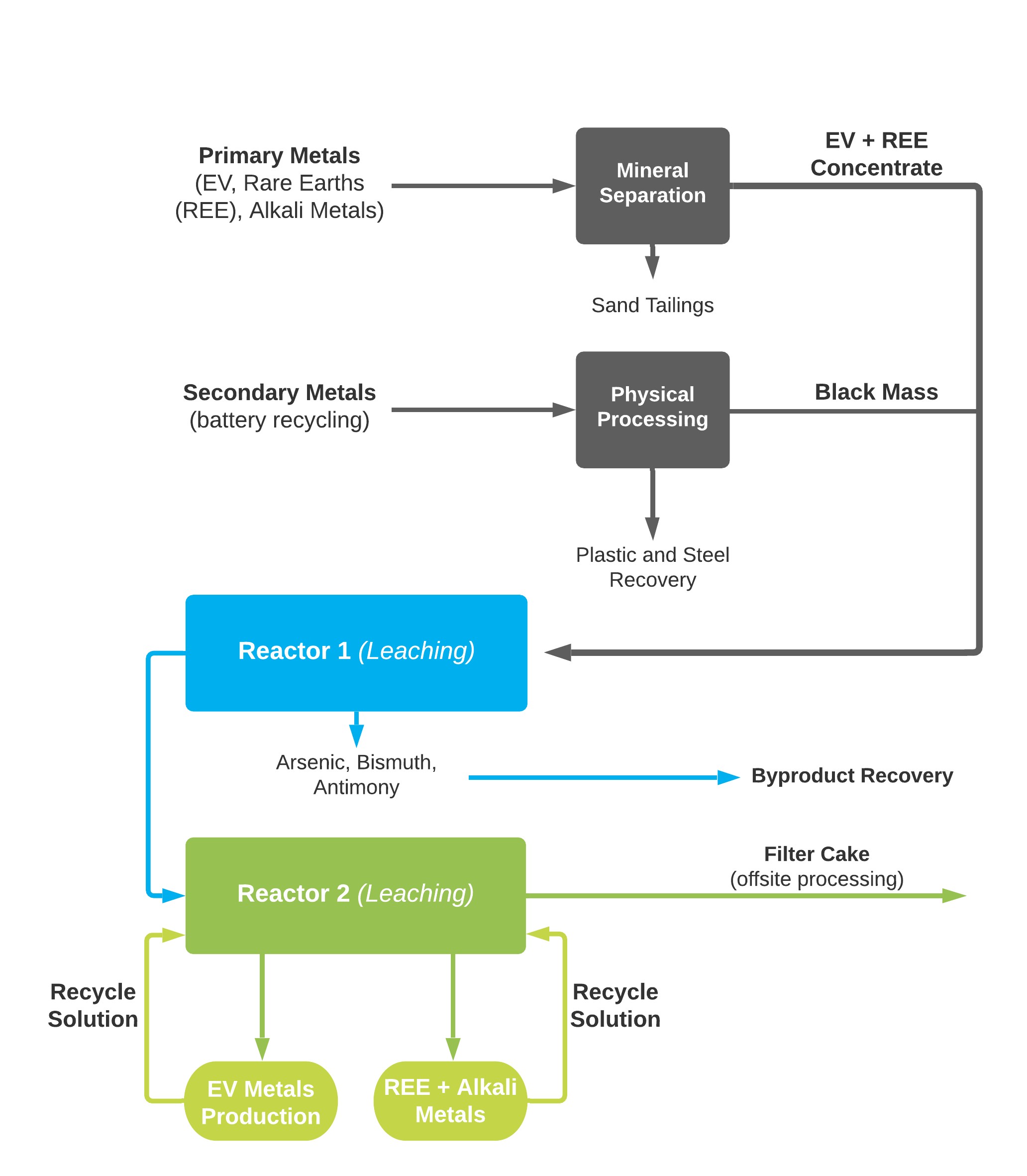
A little bit about why it's called Re-2Ox. Everybody thinks the Re means recycle. Not really. It means reactor. We have two reactors in this process that are used to take the primary metals, which are from the mines, and the secondary metals, which are the spent batteries and, of course, for the two reactors, we need oxygen in the process. We put that all into a design of a process, called Re-2Ox, as you see in the flow-sheet and our intent is to use the process to produce battery metals.
We started this about three years ago, and we wanted to recycle batteries. So we spent a fair amount of time traveling throughout Asia, North America, and Europe. And what we found was there was not actually a lot of spent batteries available. So instead of waiting for the opportunity, we created the opportunity. We created a very high cobalt-nickel-copper concentrate with high arsenic, and then we subjected it to the Re-2Ox process. And lo and behold, we produced what we call cobalt sulphate to Asian specs. It was a company called Sumitomo that gave us the specs, and they were the only ones who gave us specs, so using their specs, we did produce a cobalt sulphate for the battery market.
One of the most important things people have to understand about the Re-2Ox process is that in a lot of the recycling right now that we encountered in our travels, recyclers took the spent batteries and put them into a smelter. It's a pyro process. They basically burning it, followed then by hydrometallurgical. But with our process, there is no stack. We don't burn anything. It's all leaching. It's all hydrometallurgical.
To further explain, using our hydrometallurgical process (no smelting) we can produce a product “on spec.” If a customer comes to us and says, “Listen, we want cobalt sulphate at this grade with these impurities,” we can do that. We're going through the next stage of development now, which is where we ask SGS, a global player in this industry with 22,000 employees globally, to build us a pilot plant. Right now, what they're doing for us is they're testing three different batteries: lithium ion, nickel hydride, and nickel cadmium batteries.
What we're trying to do is establish the parameters, the bench-scale test work, to build a pilot plant. And then of course from there build a larger plant, which will produce a quantity of the battery metals. When we started in May 2018, we produced a cobalt con, which was very high in nickel and copper, and also very high in arsenic. And we were able to extract 99% of the cobalt, but also 99% of the arsenic. Now we found out the arsenic, not only is it a critical metal, it's one of the 40 metals the USA considers critical.
Then in August 2018, we produced our first cobalt sulphate from the Cobalt Camp, which I think nobody's ever produced before in the Cobalt Camp. We were very successful in doing that. And now moving forward to this year (2021), we decided we'll build a pilot plant. And we're starting to do the test work on the three different types of batteries.
What's really important, and a lot of people need to understand, is that recycling the batteries by themselves is not economical. When we looked at all these (battery recycling) processes out there, they were all smelters and the smelters had a primary metal, which is coming from their mines, and then the secondary metals, which is battery recycling, but the battery recycling was sort of secondary to the issue.
As we show in the flow chart, we do have our own primary metals that we will put through a mineral separation process, which, quite simply, produces a concentrate, which will then join with the secondary metals, which are from battery recycling. As part of the battery recycling process, we'll do a physical processing to remove the plastic and seals to produce a black mass. Then, the black mass is combined with the primary metals concentrate, and the combination goes into our first reactor.
In our first reactor, we remove what we call the economical undesirables like arsenic. When we remove the arsenic, we produce it as a cobalt arsenate, which is used in wood preservatives. From the first reactor, the material travels to our second reactor. We put all the metals into solution, and then we pick out the metals that the customer wants for their specific battery chemistry.
Critical thing to this entire process is you have to recycle the solutions. If you recycle the solutions, then of course you have what we call a green process. The Re-2Ox process is a very simple elemental process. It's hydrometallurgical, and it works with primary and secondary feeds to make the process economical and viable.
You've got to be careful when you answer this question. What we want to do is create something that's green. A smelter is not green. The economics of running a smelter, they're massive facilities, they have high volume, that's why they can do it economically.
For us to make this thing work, we have to have a primary feed, which we do have, and then we can get into the battery recycling. If we look at doing the battery recycling for batteries themselves, while our process is capable of it, we can't do it economically. We want to be competitive globally. So, the facility has to be very large to deal with the primary metals. And then of course you can do secondary metals, which are your spent batteries.
We tend to have long-term investors in our companies which are designed for the investor who likes do what is called managed risk. In the event we go into production, which we are targeting, then the big boys come in and of course, they replace the board and they say, “Frank, thank you very much. We will run this process.” Now we already met with the Germans. We also met with the Chinese. They'd like to see this process, but in reality, a process is not something you do in an afternoon. It takes a fair amount of work.
They have to see the economics and they'll go ahead and then acquire the process. So if you look at the way we've done it, you'll notice we tried to patent it. Then we found out, after talking to our patent attorneys, they said, “Frank, don't patent it, just license the process. The minute you patent it, everybody knows about it.” And some enterprise who will start using it and then all you do is have a legal battle.
So we kept the process proprietary. It is very simple, elemental. It can be built anywhere. We use old conventional reagents, nothing exotic in what we're doing. And the reality is, it's bulletproof. We spent more than six years working on this process. We've already spent 8 million dollars on it, but we still have a fair ways to go to make sure that we meet the demands of the market.
The water is repurposed, and everything is filtered. I simplified the process in the flow chart without putting making too many things in there, but yes, it is filtered. You've got to recycle the water. The water itself is valuable. So we won't throw it away. There is a great value to the water.
I think the only thing we could say is that we can do better than anybody else. We're the only ones doing it. I can take very complex high arsenic concentrate, and I found out some of the batteries also have arsenic in them, we can take the arsenic out and repurpose it. That's all.
We're the only ones doing it that we're aware of. In the old days, the smelters used to take all these arsenic cons and they would blow it out the stack. The smelters do that. There's a lot of these arsenic cons and we found a lot of them of all places in South America where they can't go anywhere to get processed. And even the Chinese, when we went to the Chinese with our con, they refused it. So we took out the arsenic and of course then they accepted it. So even the Chinese will not take high arsenic feeds.
It's a tough business. You have to find people that've been in the industry for a little while. They've been doing this for at least 40 years. There's a lot of enterprising people, very good ideas, but I tell you the recycling business, which I've been in as well, is brutal. It's not that simple. And everybody's your competition.
If you think you're gaining ahead, next thing you know, is that the battery market dries up and somebody's stockpiling batteries in Europe. And, of course, you then get no feed. So that's why we said, we're going to be a primary metal producer. We control our own feed. And if batteries show up, wonderful, we're going to toll it for them. We're not buying, but instead we're going to toll it for anybody who brings it to us. In other words, if Panasonic wants to come to us, we'll toll it for them. And we will produce product on spec.
When our team first looked at all of these processes, they were all smelter-based. The majority of the battery metals produced by those smelters were secondary. We now have our own primary metals, which we will purify using a mineral separation process.
Frank Basa
Founder & CEO
Our first reactor removes materials such as arsenic that are economically undesirable. The materials are then transported to their second reactor. Here, all the metals are dissolved into solutions from which we can extract the metals that the client requires for their specific battery chemistry.
Frank Basa
Founder & CEO

The Final Product
Pictured above is a standard electric vehicle battery pack. An unprecedented expansion in metals production will be required to meet demand regardless of which battery chemistry becomes dominant.
Find out how we can work together
We are always ready to collaborate.



